This week in AP Chemistry, we focused on state changes. We started
off with some discussion on liquids and solids, and also what it looked like
when potassium chloride was dissolved in water. Water is a polar molecule, so
when the individual K and Cl ions are broken off, they are surrounded by water
molecules and are said to be hydrated. K is a cation, so the water molecules
orient themselves so that the more electronegative O atom is pointing towards
the ion. For chloride, it’s just the opposite. The partially positive areas of
the water molecule, the H atoms, orient themselves so they are pointing towards
the chloride anion.
Soap is an everyday necessity. It’s common knowledge that soap is
able to clean the grease off of a dish But how does it remove those oils? We
discussed this question in class. Soaps are fatty acid chains with hydrocarbon
chains. It is a polar molecule, and the hydrophilic part of the chain interacts
with water through ion-dipole interactions and hydrogen bonding. The
hydrophobic parts curl up into themselves. The chains are attached by
dispersion forces and form a spherical surface, which attract the oils and fats
on a surface and store them within a sphere.
We also held a brief discussion on why humans aren’t just a mass
of liquid or gas by discussing the composition of cell membranes. Cell
membranes are made up of phospholipids, a form of fats. These phospholipids
form the lipid bilayer, and are composed of a hydrophilic head and hydrophobic
tails. Only water and gases can easily pass through this membrane, and large
molecules and small polar molecules cannot without the assistance of proteins.
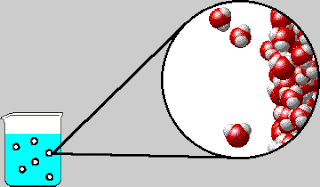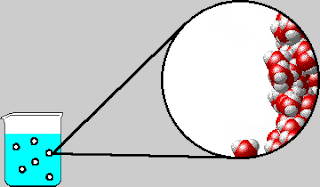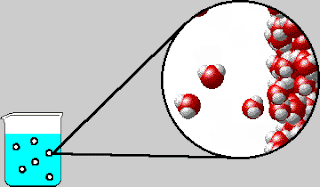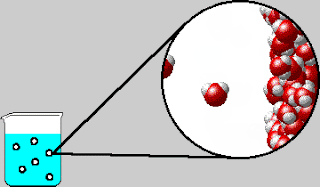
On Tuesday, we whiteboarded what was in the bubbles that come out
of boiling water. The bubbles contain water vapor, which is water in its
gaseous form. We also went over the IMF and Solids POGIL.
On Friday, we performed a minilab where we were given mystery
substances, and based on their properties, we were to decipher which substance
it was. The first part of the lab involved testing the electrical conductance
of given substances (water, ethanol, acetone, nonane, sodium chloride, steel,
and sucrose). We used conductance testers and immersed them in the liquid
states of the substances, and also tested the conductance of the dissolved and
solid states of the substances. For the substances that weren’t able to be
simulated in a certain state (such as molten steel) , we used a QR code to view
videos that tested the conductance of these substances. We found that the ionic
substance NaCl was an insulator in its solid state, but became a good conductor
of electricity when it was dissolved in water.
The second part of the minilab involved observing the properties of
different substances and trying to name them. We swirled the liquids to test
their viscosity. Glycerin was quite easy to find; it was easily the most viscous
due to its three hydroxyl groups. We also tested the evaporation rate and
surface tension of the substances, and tried to see if one substance was
soluble in another. Acetone evaporated extremely quickly due to its weak intermolecular
London dispersion forces.
This lab was very helpful in observing
how a molecule’s composition affects its properties. It all depends on the
intermolecular forces. The stronger the intermolecular force, the slower a
substance will evaporate and the more viscous it will be.

No comments:
Post a Comment