This week heralds the return of the AP Chem blogs! Much time
has past since we have had to revisit these writing assignments. We have been
exploring the topic of enthalpy and entropy (the dreaded “E” word!) and
applying them to given scenarios. We started off with discussing our
precipitation lab we performed last week. We were given a collection of mystery
solutions, and we were to determine the identity of the solutions. We mixed on
drop of one solution with a drop of another and observed the reaction; many of
them formed precipitates with varying colors. Using our knowledge of net ionic
equations and properties of precipitates, we slogged through the reactions and
eventually arrived at the correct answers.
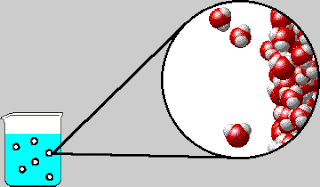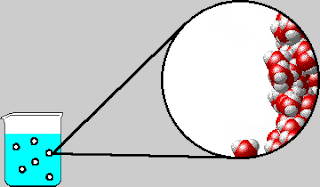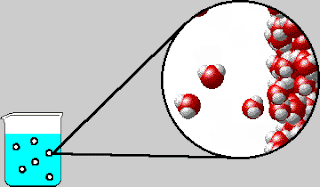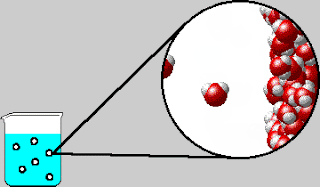
Also early in the week, we were introduced to the concept of
entropy. The elusive yet dreaded “E-word” had appeared a few times in Tri 1,
but this week is when we strove to understand the concept. Using laptops, we
completed a lesson online measuring the order of solutions and solutes.
I have confused enthalpy with entropy many times. However,
I’ve learned that enthalpy is the measure of energy in the system, while
entropy is the measure of randomness or disorder. Admittedly, entropy is still
somewhat cloudy of a concept to me; I’m still trying to understand how to
connect the disorder of a system with the available microstates of each
molecule.
In the middle of the week, we worked out the equation for
dissolving sodium acetate trihydrate through the use of standard enthalpies. It
takes 36 kJ to perform the reaction, and it is exothermic. We witnessed this
reaction when we were given heat packs; after activating them, the pack
released 36 kJ of heat, which is what we felt in our hands.
We also started a thermodynamics worksheet involving the
calculation of reaction enthalpy values using standard bond enthalpies. We
whiteboarded some problems which, as usual, proved very useful to me. The
boards did a great job of outlining the steps for finding enthalpy, and many of
my classmates asked some good questions, which helped my understanding of the
whole process.
We were also introduced to our new lab next week. We aren’t
given a procedure, just an objective and a few scraps of information. I’m
actually kind of excited for this lab; I enjoy figuring things out even though
it may be difficult for me to follow. On Friday, in fact, we worked out the
reactions for determining the heat of formation of MgO and the reaction between
Mg and HCL. Truthfully, I was having some trouble following the process, but I
understood the experiment towards the end through the help of my classmates who
were leading the process on the board. I enjoy this challenge, and I’m quite
sure 3rd hour will prevail and win the much sought-after prize: the
famed Einstein bagel!
Julien Griffith summons a ball of flame (just kidding, it's just the HCL and Mg reaction)
We also went through questions in the Entropy HotPot. We
were to read the question on the screen, and then we held up our fingers
indicating which answer we thought was correct. This proved to be inexplicably
helpful to me. I really enjoy class discussion, because many people ask
questions I myself didn’t know I had. Dr. J explained the process through which
we could answer these questions, sometimes by eliminating answers immediately
and then using logic to attack a question when we didn’t know the exact answer.
I am somewhat apprehensive towards the upcoming exams, but I’m sure the HotPots
will prove to be invaluable as a study aid.